Start: LogEntry_0002 SolYear: 8760.76
The first step to getting off this planet will have to be figuring out my propellant, basically what I'm going to continually shoot out behind me to get out of this planet's grasp, so that's where I'll begin.
I managed to decrypt some data on some guy named Bol-TZ-mann, who appears to have done quite a bit of work on the relevant mathematics and physics I would need to figure this out. He spent a lot of his time trying to understand the complexities of a moving gas, without understanding the individual movement of every single particle, which lead to his contributions to statistics.
I've luckily found a way to make pure hydrogen gas(H2), so I can simplify my life by using an ideal gas model, this is because H2 gas has very very very weak intermolecular attractions, and because the molecules are incredibly small, and thus unlikely to crash into each other.
Before I can use Bol-TZ-mann's work to escape this planet's grasp, I have to understand his work, which I hope I will manage.
He described, among other things, how random things in our natural world, will tend to have a certain value, most things will have a value close to this certain value, even though few things will have that exact value.
This Bol-TZ-mann discovered how one can describe this phenomenon mathematically, I won't explain how he derived this formula, as it is not necessary to use it, but I will explain what goes into it. The formula is \(P(x) = \frac{1}{\sigma \sqrt{2\pi}} e^{- \frac{1}{2} \big( \frac{x - \mu}{\sigma} \big)^{2} }\), this formula may seem daunting and complicated at first, but it is surprisingly simple! The value \(P(x)\) is the probability density around the point \(x \), \(\mu\) is the value I'm centered around(also know as mean/average value), and finally \(\sigma\) is how far away from the \(\mu\) value I can find certain amounts of these values(also known as standard deviation).
The term probability density might seem a bit weird at first glance, how can one have a density of probabilities? Let's say I'm looking at some measurement of length, it does not really matter what length measurement I'm looking at(say we're looking at finger length if you want), and then I solve the above equation taking into account it's units, I end up with a final unit for \(P(x)\) of \(m^{-1}\), which means I can read it as probability per unit metre. To get this into pure a "pure" probability I'm going to have to multiply \(P(x) \) with some distance \(\Delta x\), to get a value as close to \(x\) as possible I will have to choose a \(\Delta x\) as small as possible, so I choose an infinitesimal(Infinitely small) \(\Delta x\) so that I can call it \(dx\) instead. Now if I want to find the probability of finding a length measurement within the range between \([ x, x+dx ]\), I will use the equation \(P(x) dx\), pretty neat if you ask me!
I can actually recognise that this reminds me of integrals, and I can write it on integral form! This means that I get \(\int_{a}^{b}P(x)dx\), which will then give me the probability of finding a measurement within the range \([a,b]\), which I now can set to whatever I want!
I should also note that this does not give me probabilites in percentages, it gives me values between 0 and 1, where 0 is 0% probability and 1 is 100% probability. Also to take note of: \(\int_{-\infty}^{\infty}P(x)dx = 1\), this means that if I want to find the probability of finding a value within all possible values, that probability is 100%. Finally, this function gives us what is known as a normal or Ga-Uss-ian distribution, to use the correct terminology.
With that theory hopefully understood(by me that is), back to the gas I want to use to propel me out of this! It turns out this Ga-Uss-ian distribution does not describe how "fast" every particle in a gas moves, this is however not a problem, since how "fast" a particle moves is usually a measure of how fast it moves in one direction, and does not take into account how fast it moves in different spatial directions(x,y,z for example). The Ga-Uss-ian distribution does however describe the velocities of particles in the spatial directions, which means I can use this to simulate how the particles move about and work from there!
This Bol-TZ-mann also found worked out some very useful things for how this distribution will look for ideal gases, namely the standard deviation \(\sigma\) and average velocity \(\mu\) in one spatial dimension. For an ideal gas where every particle has mass \(m\) and temperature \(T\), the standard deviation will have a value \(\sigma = \sqrt{\frac{k_{B}T}{m}}\), where \(k_B\) is a constant named after this Bol-TZ-mann character. The average velocity \(\mu \) does not have an equation like the standard deviation, because I can simply reason that \(\mu = 0 \frac{m}{s}\). If this doesn't make sense, remember that I'm looking at one spatial dimension only, and that we should be equally likely to have a positive or negative velocity, or rather for any particle we're looking at it is equally likely to move "forwards" as it is to move "backwards".
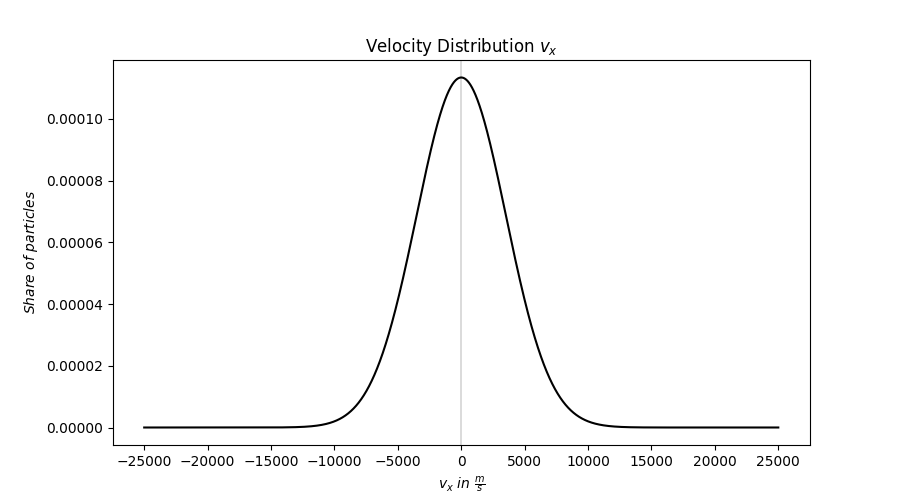
I've made my robots make a plot to illustrate the velocity distribution along one spatial dimension.
While I've only argued for one spatial dimension thus far, it should be easy to understand that these things will be true in all spatial dimension.
Allright, now I've got a grasp on what velocities the particles of my gas should have, I've yet to address where they should be, statistically speaking. I've spent some time pondering this, and I can't think of a reason any particle is more likely to be in any one position, than any other position. They're just flying about randomly, with velocities depending on their their temperature (and mass), and are just as likely to be in position A as in position B. I feel pretty confident that I can just use a regular uniform distribution when placing my particles.
I just realised that I've completely forgotten to explain what I'm going to use all of this statistics work for, silly me, the solitude has made me quite forgetful!
As mentioned before, I'm trying to figure out how to use my propellant to escape this planet's grasp, and to do this I have to figure out how the particles of my propellant will behave under pressure. So what I'm going to do is simulate a tiny box, for some hundred thousand particles, and figure out if Bol-TZ-manns theoretical work holds up to my numerical simulations.
Well, time to end this log entry, I've got to get back to improving my manufacturing and exploring the local area.
More details can be found here
Oh and finally, I've made some progress on my factory!

End: LogEntry0002