An attractive vaccine strategy against gonorrhea is immunization with outer membrane vesicle (OMV) vaccines because they have the potential to elicit an immune response against the conformational correct components of the bacterial surface, and the strains can be genetically modified to express additional or variant antigens. Relevantly, a meningococcal OMV MenB vaccine, MeNZB developed at NIPH, has been shown to have 31% effectiveness against gonorrhea in New Zealand, suggesting that efficient vaccination against gonorrhea is feasible. Several antigens present in OMV-based MenB vaccines are conserved across N. meningitidis and N. gonorrhoeae, but the mechanism by which the OMV vaccines provide conceivable cross-species efficacy is not fully understood.
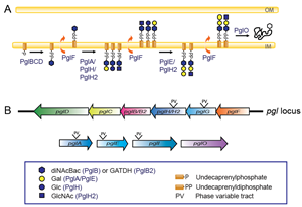
A) Glycosylation pathway
B) Protein glycosylation (pgl) genes
Species of Neisseria express broad-spectrum O-linked protein glycosylation where the glycan is attached to serine (1-3). A simplified overview over the glycosylation pathway and involved pgl genes in N. gonorrhoeae are presented in the figure. Significantly, we have previously established that these glycoforms are antigenic and immunogenic (1). They are often antigenically variable and subject to phase variable (on-off) expression. Individual gonococcal strains can simultaneously express 1-5 glycoforms, while the total within-species glycan antigenic repertoire identified is currently 6 major glycoforms (4-6). Importantly, our unpublished data show that glycan specific antibodies in human sera are bactericidal.
Master projects:
I. Glycan antigen distribution in strains of N. gonorrhoeae
In many ways, pgl glycans fulfill key criteria for an optimal vaccine antigen in that they are surface-exposed and possess limited antigenic variability. This master project will perform a systematic approach to decipher the full glycan antigen distribution in strains of N. gonorrhoeae and for complete coverage in our proposed OMV vaccine. The student will investigate available N. gonorrhoeae whole genome sequences (WGS) to select a representative collection that contains both phylogenetic distal isolates and pgl gene variants. Next, pgl gene analysis and glycosylation phenotyping will be performed with the intention to discover strains with novel glycan antigens and predict glucan antigen distribution.
This project includes the following techniques:
- Whole genome sequencing and bioinformatics analysis of N. gonorrheae strains to identify the protein glycosylation genes
- Immunoblotting of N. gonorrhoeae strains against glycan specific antibodies
- Correlation of pgl genotype and glycosylation phenotype
- Possibly investigate the strains associated with novel glycoforms, to characterize the genetics in detail. First, the different pgl genes will be knocked out in the wild type strain to decompose the glycan. Then, the glycan will be reconstructed in a previously well-characterized strain by exchanging the relevant pgl genes and/or by introduce pgl gene mutations.
II. Immune responses in available material and immunological role of glycan specific antibodies
We have available sera (human and mice) from several previous OMV immunization (7-8) and the project will investigate to what extent OMV immunization engender antibodies against glycan antigens. In addition, we want to investigate the protection offered by these antibodies through antibody functional assays
This project includes the following techniques:
- Immunoblotting of mice sera against a total panel of well-defined glycan expressing strains to identify glycan specific antibodies.
- Serum bactericidal activity (SBA) assay. Bactericidal activity of serum antibodies (activation of the complement system leading to lysis of the bacterial cell) is known to correlate with protection against N. meningitidis. Although correlates of protection against gonococcal infection have not been defined in the same manner as against meningococcal infection, there is evidence that complement-mediated killing may also predict gonococcal vaccine efficacy. SBA assays with wt and mutant N. gonorrhoeae strains will be performed.
- Human cell adherence assay. Type IV pilus is the primary mediator of N. gonorrhoeae adherence to human epithelial cells and colonization of human mucosal tissue and is heavily glycosylated. The project will assess the potential for glycan specific antibodies to bind to the pilus and influence adherence of the bacteria
Supervisors:
The project will be performed at the Norwegian Institute of Public Health.
Bente Børud, senior researcher at the Norwegian Institute of Public Health, will be supervisor and Mike Koomey, professor at IBV, will be co-supervisor.
Referanser:
1. Børud, B et al. J Bacteriol 192, 2816-2829 (2010).
2. Vik, A et al. Proceedings of the National Academy of Sciences 106, 4447-4452 (2009).
3. Anonsen, JH et al. J Proteome Res 11, 5781-5793 (2012).
4. Aas, FE et al. Mol Microbiol 65, 607-624 (2007).
5. Børud, B et al. Molecular Microbiology 94, 688-699 (2014).
6. Børud, B et al. Proceedings of the National Academy of Sciences 108, 9643-9648 (2011).
7. Liu, Y et al. Mucosal Immunology 10, 1594-1608 (2017).
8. Sandbu, S et al. Clin Vaccine Immunol 14, 1062-1069 (2007).