Mission control has requested we sample the atmosphere, and check its contents prior to landing. This is apparently important so we don't "Burn up like cousin furgie", so I guess we'll get on it.
Firstly we'll be simplifying the atmosphere into a shallow rippoff of an ogre; like an onion with two layers. These layers are the Isotherm and adiabatic layers. We won't go into much detail on these, just know that in the isotherm layer, the upper part of the atmosphere, the temperature is constant with height and therefore decreases exponentially upward. While in the adiabatic layer, which is around the surface, there is no transmission of heat. We'll also be assuming the gass is at a hydrostatic equalibrium meaning the atmosphere is relatively stable compared to the planet surface. In other words; it won't collapse to the planet surface, and it won't drift off into space. We aslo assumen an equal distribution of the gasses found in the atmosphere. If we have two gasses we have a 50/50 split, if we have five gasses we'll assume the atmosphere is made of 20% of each.
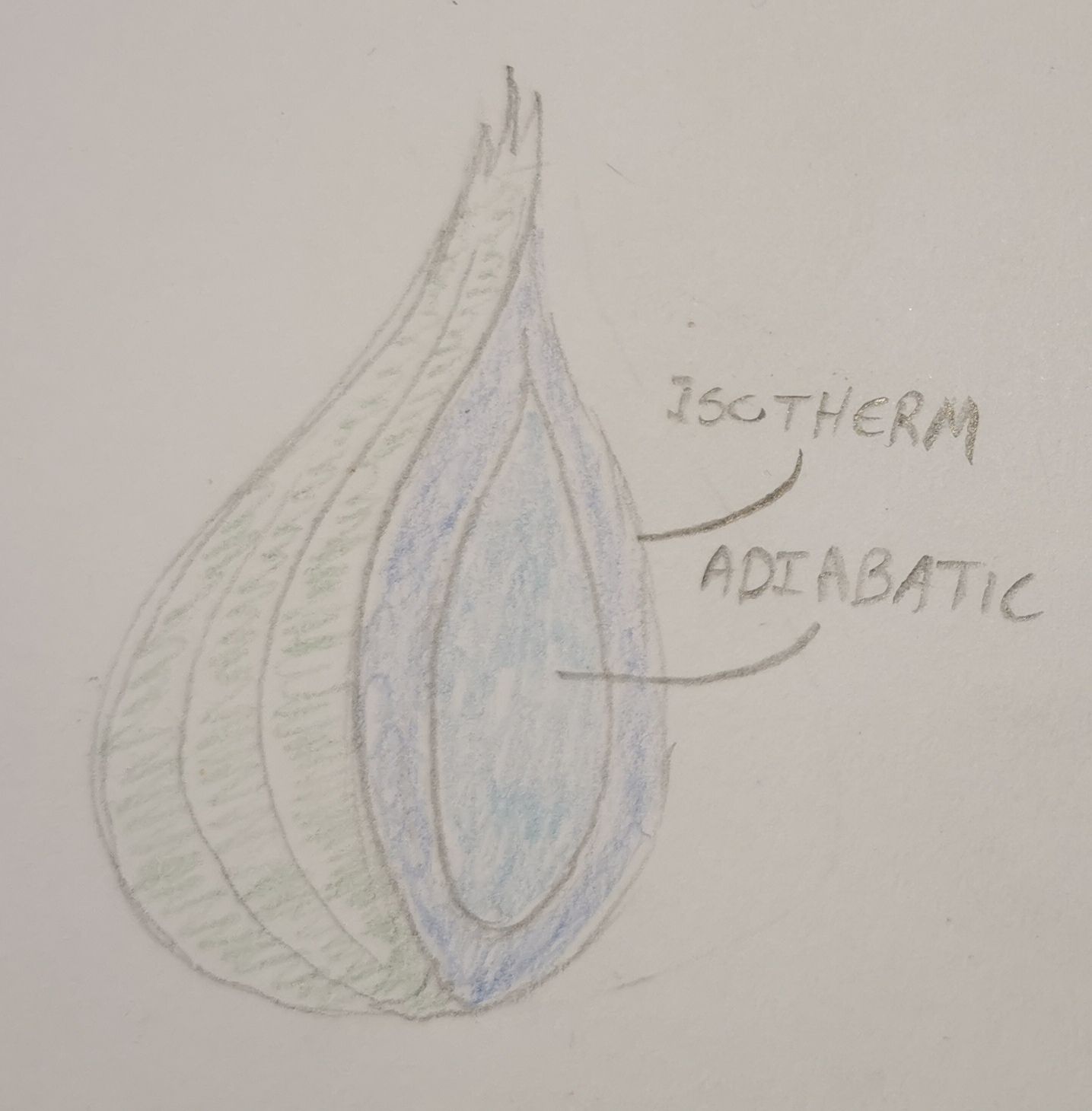
The rationale behind spending time on this, is in order to make a simple model of how the atmosphere will behave during landing. In practice we're figuring out how effective our parrashoot will be during landing, the maximum speed we can achieve before the friction forces from the atmosphere burn us up, and what the thermal velocity is (which we will talk about in a future post). If we don't do this we'll either end up as a glorified crater on the planet surface, or as a shishkebab piercing the sky. You can probably reason your way to this, but different contents of gass in the atmosphere will usually quite drastically change its behavior with respect to wind and friction, this is why it is important for us to know what is in it.
Now onto how we find these substances. You may have seen a couple of pictures like the one to the left in school, these are so-called spectral lines, the coloured lines on a black background are emission lines, these are caused by gas being heated way up and exiting electrons in the atoms. This is more relevant when looking at stars, so we'll look at the absorption lines, which are created when light travels through gass. What happens when the light travels through, is the light of specific wavelengths exites electrons, and is then emitted in a random direction once the electrons return to their base position. And since each molecule has different energy levels we can assign particular molecules to particular sets of lines. This is the concept we will be using to identify the molecules in the atmosphere of Lajaland, but we have a couple of challenges to take into account here...
Since the molecules have pretty distinct indicators on a spectral line finding them should be a breeze, right? Well... we have a teeny tiny problem known as noise (link to noisy stars) which we covered a bit earlier. This essentially means our measurments are scuffed and sometimes very wrong, but what specificly gets janked up here? One exaple is our motion around the planet. Our velocity is fairly high; a few kilometres a second, so we expect to see the gasses in the atmosphere reflect a similar velocity compared to us, with some minor deviation due to wond and such. However, due to noise this can mean we get readings that tell us the gass in moving at a completely different velocity from what is should.
We get around this by making a few assumptions, and looking at multiple properties of the gass, think of it as a checklist. If the gass checks out on all points, within a slight deviation we should see it in our atmosphere, if it's for example moving at the velocity of the spacecraft, but in the opposite direction, i.e. twice as fast as the spacecraft, we can reasonably assume it is not in the atmosphere. Either that or we're in for some crazy turbulence upon landing

Velocity aside, we should see gass of the same veriety have around the same temperature on the planet. We expect this to be the case since it's abnormal to have something like an area of the surface that's freezing cold showers directly next to one with scalding hot damp, in the case of water. This concept applies to most gasses, we can also relay this back to the adiabatic layer, as heat won't really escape the atmosphere here, it should distribute reasonably evenly though gasses of the same type, between different kinds of gass, however, we expect to see some variation as different gasses require different amounts of energy to heat up. We also expect the gasses to be somewhere between 150K-450K, as otherwise we'd probably read a slightly higher temperature of the planet surface than we did earlier.
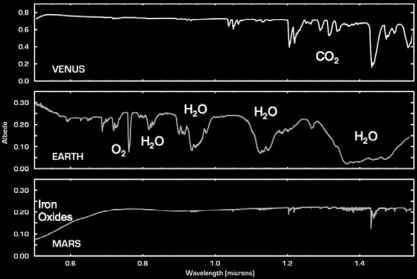
Next we'll have a look at absorption line; to the left you can see a bunch of spectral lines for the sol-solar system. The dips in the graph mark points where the wavelengths in the flux (light reflected from the planet, hitting our spacecraft) is absorbed. For water, which is kinda important to us, these dips should occur around 632, 690 and 760nm, so we maily look for dips around these wavelengths, so we don't need to analyse litterally thousands of different irrelevant wavelengths. The wavelength, without the noise disruptiong it should dip to about 70% of the light normally reflected off the surface, but we need to give some leeway in case there's a really bad reading here.
When we consider all the above; velocity compared to spacecraft, temperature consistency within the same gass, temperature range and the deviation at each wavelength, we can make an educated guess as to which gasses exist in our atmosphere. But how do we do this you ask, well my pupils first you must open your sences to the cosmos, feel the galactic wind brush against your cosmic boosom, for I will show you the way of chi...
The method of least \(\chi^2\) (Chi squared) is very similar to the method of least squares, but it has one key distinction; the \(\sigma\) varies with every measurment. You can probably see the similarity: \(\chi^2=\sum\limits_\lambda(\frac{F^{obs}(\lambda)-F^{modell}(\lambda)}{\sigma_{noise}(\lambda)})^2\). The goal here, like earlier, is to get the deviation as close to zero as possible. In order to minimise the effect the noise has on our measurements. Though it is preferable to have a few to many error readings. The reason for this is so we don't accidentally filter out realistic options where the \(\sigma_{noise}\) was especcially high. Since errors that don't fall on the desired wavelengths can easily be disguarded as we know none of the molecules fall within this wavelength. On the other hand, if we had too strict criteria we may have a wavelength of say oxygen end up not being included, although it matched the temperature and velocity criteria, but just barely falls outside our desired range on wavelength requirement. Now all that stands between us and a cool onion-based atmosphere model is finding our gasses :ok_hand_emoji:
